The United States Food and Drug Administration (FDA) has defined Process analytical technology (PAT) as a mechanism to design, analyze, and control pharmaceutical manufacturing processes through the measurement of Critical Process Parameters (CPP) which affect Critical Quality Attributes (CQA).
Traditionally, pharmaceutical production has involved the manufacture of products using batch processes with subsequent laboratory testing and analysis to verify quality. Modern process control engineering and manufacturing achieves higher quality and reduced costs through the use of on-line process monitoring and control. In addition, these methods offer the pharmaceutical industry the opportunity to lower product cycle times and reduce variability.
To enable process control, NIR and FTIR techniques are frequently used to monitor component concentrations in API formulations. However, excipient interferents limit the dynamic range of measurement and these techniques are typically limited to powder mixtures >2% bulk ratio.
Deep UV Raman and photoluminescence or fluorescence optical methods offer the ability to detect and identify material at much lower concentrations with higher specificity than is possible with near-UV, visible, or IR methods due to the higher energy photons associated with UV excitation. At longer excitation wavelengths many materials are simply not detectable at all using Raman or fluorescence optical methods. Excitation below 250 nm enables fluorescence-free Raman where the spectral regions of Raman and fluorescence occur in different spectral regions and there is no overlap and resulting interference. Since fluorescence is so much more efficient than Raman emissions, the slightest amount of fluorescence from an API or anything surrounding the API within the laser beam excitation spot can easily obscure Raman emissions. In addition, excitation below 250 nm also enables fluorescence detection of small organic molecules not possible with excitation at longer wavelengths since these molecules, including many pharmaceutical APIs, emit at wavelengths as short as 270 nm and would not exhibit fluorescence if excited at longer wavelengths.
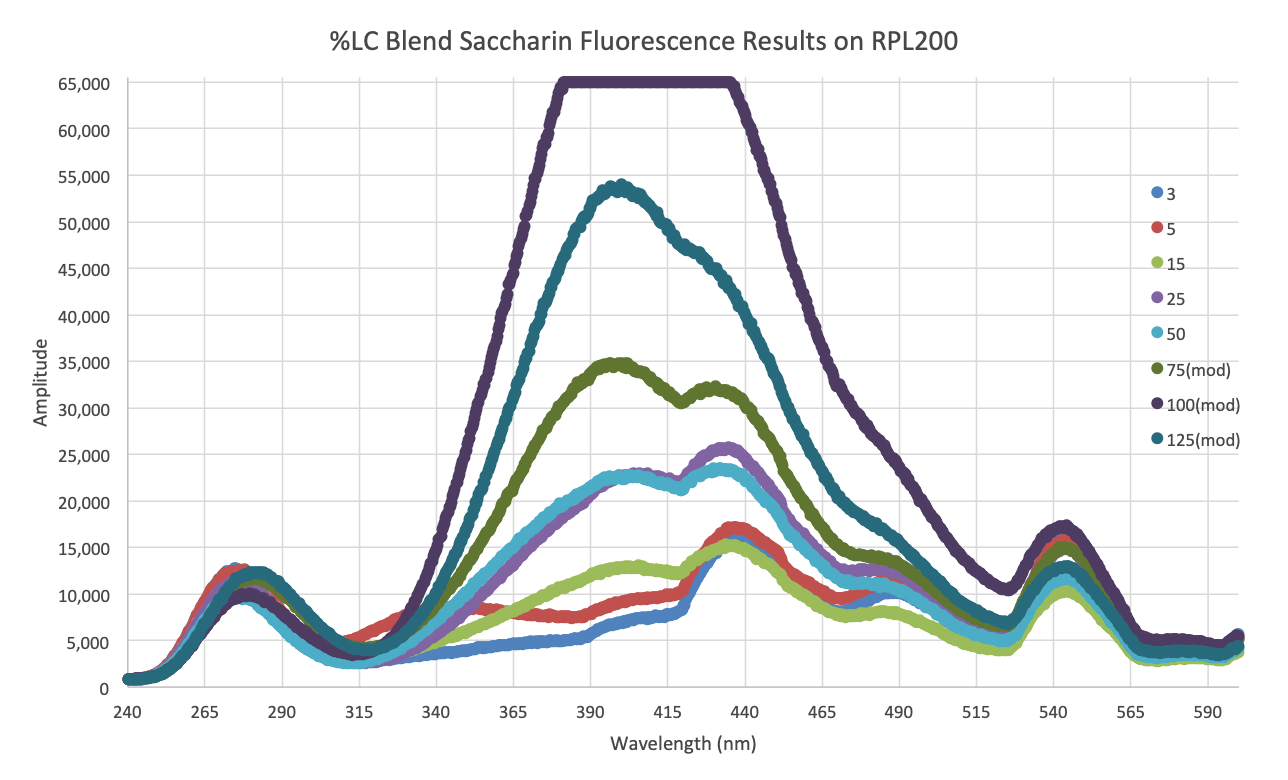
Measurement techniques based on deep UV fluorescence and Raman analysis have the potential to monitor and control mixtures to better than 0.1% bulk ratio.
Deep UV (<250nm) excitation separates the Raman and fluorescence spectral regions and allows the simultaneous detection of Raman and fluorescence. Fluorescence is the most sensitive method of detection, with over 10e6 times more sensitivity than Raman. This provides longer standoff distances and/or detection at lower concentrations.
Raman provides information about chemical bonds and functional groups, including those that do not fluoresce (aliphatics and simple compounds) Fluorescence data provides information about the overall electronic structure of target & substrate components (aromatics, ketones, aldehydes).
Key Issues
Real Time Measurement
High Sensitivity
High Specificity
Ability to Monitor Low Bulk Ratios
Downloads
DUV Raman PL 200
A fully integrated lab model deep UV resonance Raman, fluorescence and photoluminescence spectrometer with microscopic imaging.
TraC
The TraC can be fitted with liquid samplers, flow cells, or windows to look directly into mixing or bioreactor growth chambers process monitoring and for product quality control
Get In Touch
Keep informed about the latest deep UV developments at Photon Systems by joining our mailing list.
ADDRESS
1512 Industrial Park St. Covina, CA 91722-3417
PHONE
626 967-6431


