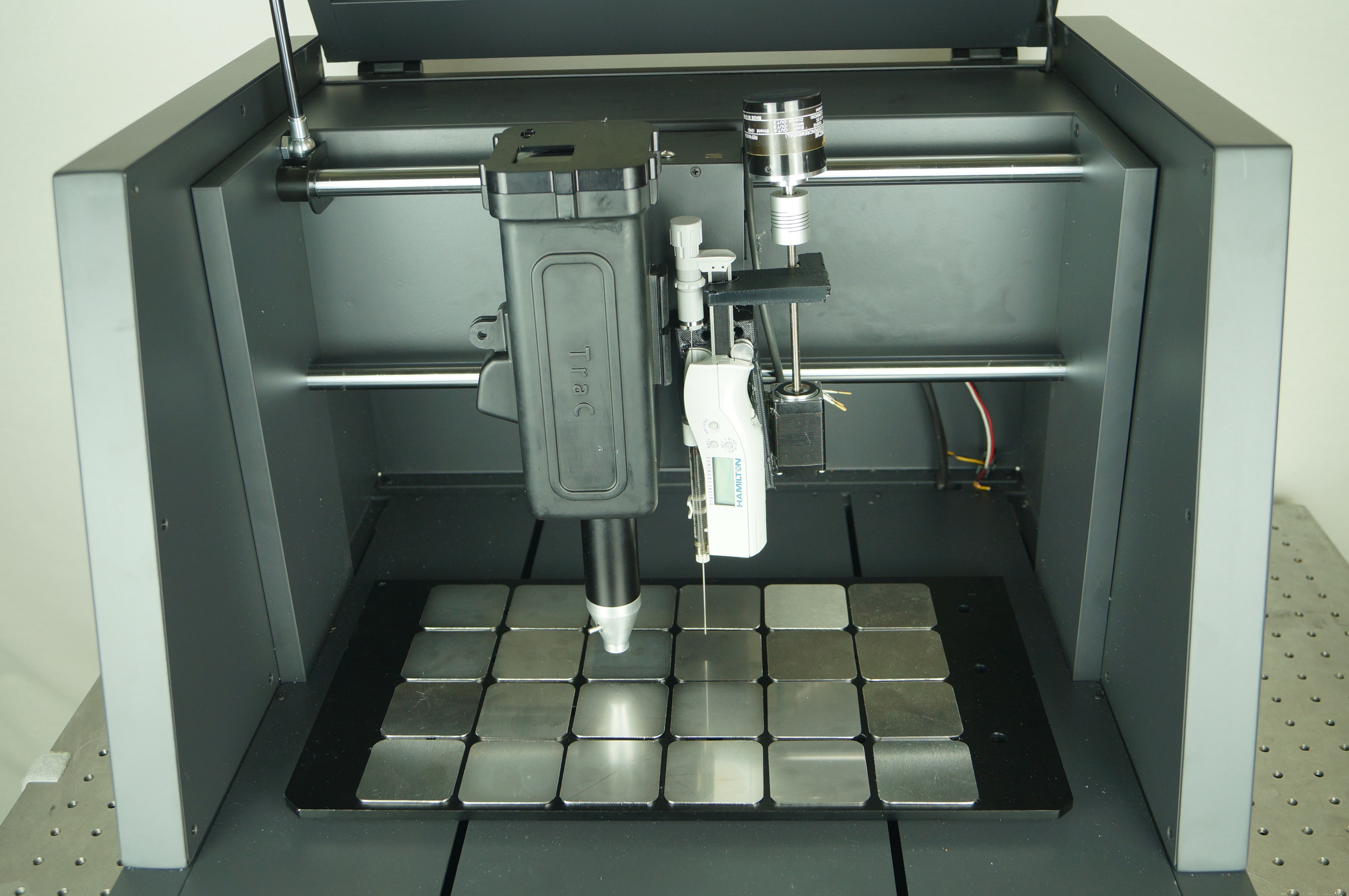
The ChemCal chemical printer is an instrument with two functions. The first function is to generate precisely known concentrations of single or multiple chemical compounds on smooth or irregular surfaces. This provides well defined chemical targets for calibrating detectors used for measuring the type and concentration of trace chemical contaminants on surfaces. The second function of the ChemCal is to calibrate the TraC trace chemical detector over a wide range of chemicals and chemical concentrations. The ChemCal enables the automatic generation of a chemical concentration curve.
The ChemCal can handle as many as 20 different chemicals, which can be laid down on both smooth and irregular surfaces in a variety of nanodroplet patterns, which are interleaved or overlaid to suit the chemical detection application. Colloidal dispersions, such as microbial dispersions can also be accurately deposited. In addition, the ChemCal can handle a wide range of fluid viscosities and surface tensions.
The advantage of ChemCal over other multi-nozzle inkjet array methods is that ChemCal can deposit highly accurate concentrations of materials in both uniform and non-uniform concentrations or gradients to simulate situations in which trace contamination detectors are employed.


Key Features
Surface Chemical Printer
Produces known chemical distributions and precise droplet mass deposits.
Wide Range of Substrates
Chemicals can be dispersed on a wide range of substrate surface materials and topography.
Chemical Mapper
Calibrates the TraC over a wide range of chemicals and chemical concentrations.
Broad Areal Concentration Range
<1ng/cm2 to >10mg/cm2
Downloads
TraC
The TraC is calibrated by the ChemCal over a wide range of chemicals and chemical concentrations.
Pharmaceutical Cleaning Validation
Detecting and quantifying trace residue concentrations of active pharmaceutical ingredients (API), excipients, and washes during cleaning validation procedures on pharmaceutical manufacturing equipment is one of the single largest costs associated with the manufacture of pharmaceutical drugs.
Food Equipment Cleaning Validation
Traditional methods of food equipment cleaning validation include microbial assays and visual inspection. However, these techniques have limitations. Using deep UV fluorescence techniques, Photon Systems handheld detectors are capable of detecting trace organic residues as low as pg/cm2 and biological contamination less than hundreds of cells or spores.
Get In Touch
Keep informed about the latest deep UV developments at Photon Systems by joining our mailing list.
ADDRESS
1512 Industrial Park St. Covina, CA 91722-3417
PHONE
626 967-6431



